AI "Discovers" Lithium from Hydrogen and Sodium
A Demonstration Towards Intelligent Modeling in Quantum Physics
In a landscape dominated by innovation, it seems a journey back to basics is what sometimes sets the stage for the most mind-bending advancements. This appears to be the case with a recent development in the application of artificial intelligence to quantum physics.
Artificial intelligence, or AI, has rapidly transformed various facets of our lives, from voice recognition and image processing to forecasting financial markets and making medical diagnoses. But in the complex world of quantum physics, the leap forward has just started.
Scientists have been deploying AI to unlock the complexities of the atomic world for quite some time now. They have been particularly interested in modeling the potential energy surface (PES) - a vital concept that represents the energy of a system as a function of the positions of all its atoms. The PES is the Rosetta Stone of atomic systems, holding the key to understanding the system's properties and behaviors.
Now, researchers have put forward a Deep Potential model with a novel attention mechanism, called DPA-1, which shows promise in learning and interpreting the PES. It's the interpretability part of this equation that recently gave us an unexpected surprise.
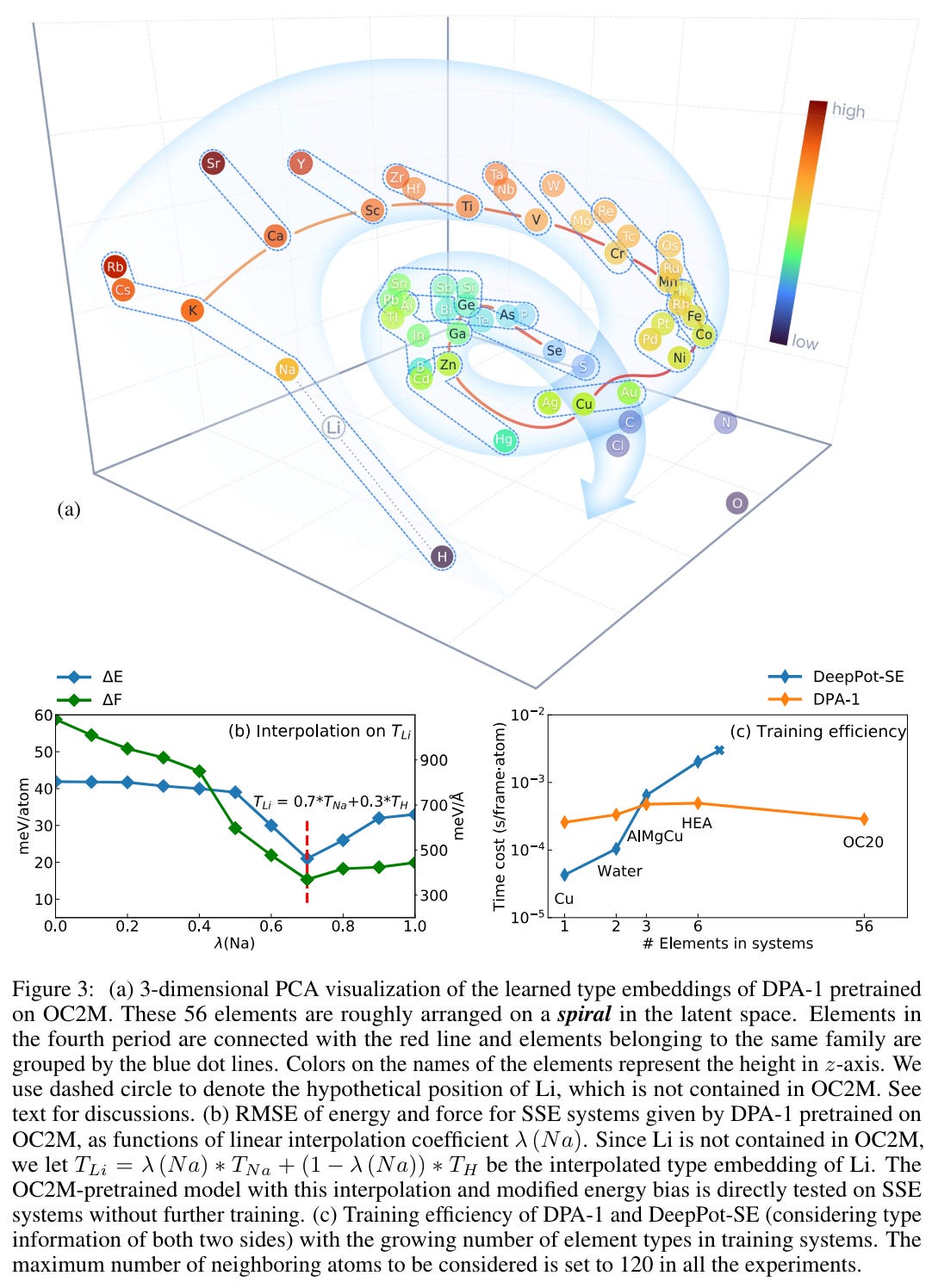
The researchers trained the model on various atomic systems but left out one particular element - Lithium (Li). In a captivating twist, they decided to use the AI model to "re-discover" lithium, an element sitting between Hydrogen (H) and Sodium (Na) on the periodic table.
To do this, the researchers generated a new representation for lithium by blending the AI's learned representations of hydrogen and sodium. They then adjusted the blend until the model's performance significantly improved. Astonishingly, this occurred when the representation was 70% sodium and 30% hydrogen, in line with the atomic properties of lithium in relation to sodium and hydrogen.
This "re-discovery" of lithium is significant for several reasons. For one, it illustrates the model's ability to transfer what it has learned about certain elements to others it has not encountered before - a trait known as transferability. Secondly, it provides insights into the internal workings of the AI model, showing that the learned representations are not arbitrary but correspond with the structure of the periodic table - a characteristic known as interpretability.
Finally, it offers a proof of concept for the use of AI to pretrain on a broad dataset and then fine-tune on specific tasks, a strategy that has become increasingly common in machine learning applications. This combination of transferability, interpretability, and pretraining and fine-tuning capabilities is like hitting a trifecta in the AI race.
Is this a whimsical quirk of serendipity or a significant breakthrough? While the experiment is captivating, it's just one piece of the puzzle. The real test lies in the model's ability to generalize these findings to other unseen elements and across different types of tasks and datasets. And while this AI experiment doesn't advance our understanding of quantum physics per se, it certainly illuminates how we can leverage AI models to learn, represent, and apply domain-specific knowledge in powerful ways.
Like the most inspiring scientific journeys, our re-discovery of lithium through AI is just a beginning. The spiral of innovation continues, and as we delve deeper into the atomic realm, the true potential of AI in quantum physics is yet to be discovered.